
What Is A Fuel Cell?
Where does the hydrogen come from?
Generating Hydrogen
Fossil Fuel Based Hydrogen Production
Water Based Hydrogen Production
Other Methods of Hydrogen Generation
How does a fuel cell work?
Fuel Cell Benefits
Environmental Benefits
Engineering Benefits
Domestic Energy Security
Independence from the Power Grid
Fuel Cells vs. Traditional Batteries
Types of Fuel Cells
Phosphoric Acid
Proton Exchange Membrane or Solid Polymer
Molten Carbonate
Solid Oxide
Alkaline
Direct Methanol Fuel Cells
Regenerative Fuel Cells
What Is A Fuel Cell?

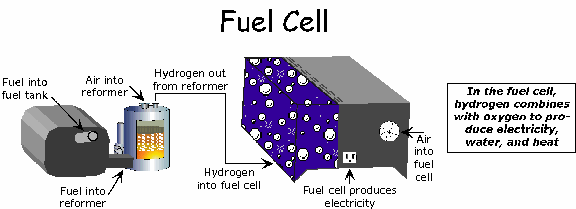
In principle, a fuel cell operates like a battery. Unlike a battery, a fuel cell does not run down or require recharging. It will produce energy in the form of electricity and heat as long as fuel is supplied.
A fuel cell consists of two electrodes sandwiched around an electrolyte. Oxygen passes over one electrode and hydrogen over the other, generating electricity, water and heat.
Hydrogen fuel is fed into the "anode" of the fuel cell. Oxygen (or air) enters the fuel cell through the cathode. Encouraged by a catalyst, the hydrogen atom splits into a proton and an electron, which take different paths to the cathode. The proton passes through the electrolyte. The electrons create a separate current that can be utilized before they return to the cathode, to be reunited with the hydrogen and oxygen in a molecule of water.
A fuel cell system which includes a "fuel reformer" can utilize the hydrogen from any hydrocarbon fuel - from natural gas to methanol, and even gasoline. Since the fuel cell relies on chemistry and not combustion, emissions from this type of a system would still be much smaller than emissions from the cleanest fuel combustion processes.
Fossil Fuel Based Hydrogen Production
A closer look at the chemical formula for any fossil fuel reveals that hydrogen is present in all of the formulas. The trick is to remove the hydrogen safely, efficiently and without any of the other elements present in the original compound. Hydrogen has been produced from coal, gasoline, methanol, natural gas and any other fossil fuel currently available. Some fossil fuels have a high hydrogen to oxygen ratio making them better candidates for the reforming process. The more hydrogen present and the fewer extraneous compounds make the reforming process simpler and more efficient. The fossil fuel that has the best hydrogen to carbon ratio is natural gas or methane(CH4).
Steam Reforming of Natural Gas
Hydrogen production from natural gas commonly employs a process known as steam reforming. Steam reforming of natural gas involves two steps. The initial phase involves rendering the natural gas into hydrogen, carbon dioxide and carbon monoxide. This breakdown of the natural gas is accomplished by exposing the natural gas to high temperature steam. The second phase of steam reforming consists of creating additional hydrogen and carbon dioxide by utilizing the carbon monoxide created in the first phase. The carbon monoxide is treated with high temperature steam and the resulting hydrogen and carbon dioxide is sequestered and stored in tanks. Most of the hydrogen utilized by the chemical and petroleum industries is generated with steam reforming. Steam reforming reaches efficiencies of 70% - 90%. The reformer component on a complete fuel cell system is usually a smaller variation of the process described above. Component reformers operate under varying operating conditions and the chemical path that the hydrogen generation follows will vary from manufacturer to manufacturer, but the resulting hydrogen reformate is essentially the same.
Water Based Hydrogen Production
-
Electrolysis
Electrolysis is the technical name for using electricity to split water into its constituent elements, hydrogen and oxygen. The splitting of water is accomplished by passing an electric current through water. The electricity enters the water at the cathode, a negatively charged terminal, passes through the water and exists via the anode, the positively charged terminal. The hydrogen is collected at the cathode and the oxygen is collected at the anode. Electrolysis produces very pure hydrogen for use in the electronics, pharmaceutical and food industries.
Photoelectrolysis, known as the hydrogen holy grail in some circles, is the direct conversion of sunlight into electricity. Photovoltaics, semiconductors and an electrolyzer are combined to create a device that generates hydrogen. The photoelectrolyzer is placed in water and when exposed to sunlight begins to generate hydrogen. The photovoltaics and the semiconductor combine to generate enough electricity from the sunlight to power the electrolyzer. The hydrogen is then collected and stored. Much of the research in this field takes place in Golden, Colorado at the National Renewable Energy Laboratory.
Photobiological
Photobiological production of hydrogen involves using sunlight, a biological component, catalysts and an engineered system. Specific organisms, algae and bacteria, produce hydrogen as a byproduct of their metabolic processes. These organisms generally live in water and therefore are biologically splitting the water into its component elements. Currently, this technology is still in the research and development stage and the theoretical sunlight conversion efficiencies have been estimated up to 24%. Over 400 strains of primitive plants capable of producing hydrogen have been identified, with 25 impressively achieving carbon monoxide to hydrogen conversion efficiencies of 100%.
In one example, researchers have discovered that the alga, Chlamydomonas reinhardtii, possesses an enzyme called hydrogenase that is capable of splitting water into its component parts of hydrogen and oxygen. The researchers have determined the mechanism for starting and stopping this process, which could lead to an almost limitless method for producing clean, renewable hydrogen. The algae need sulfur to grow and photosynthesize. Scientists found that when they starved the algae of sulfur, in an oxygen-free environment, the algae reverted to a hydrogenase-utilizing mode. This mechanism was developed over millions of years of evolution for survival in oxygen-rich and oxygen-free environments. Once in this cycle, the algae released hydrogen, not oxygen. Further research is necessary to improve the efficiencies of the engineered plant systems, collection methods and the costs of hydrogen generation.
Where does the hydrogen come from?
Hydrogen made from renewable energy resources provides a clean and abundant energy source, capable of meeting most of the future's high energy needs. When hydrogen is used as an energy source in a fuel cell, the only emission that is created is water, which can then be electrolyzed to make more hydrogen – the waste product supplies more fuel. This continuous cycle of energy production has potential to replace traditional energy sources in every capacity – no more dead batteries piling up in landfills or pollution-causing, gas-guzzling combustion engines. The only drawback is that hydrogen is still more expensive than other energy sources such as coal, oil and natural gas. Researchers are helping to develop technologies to tap into this natural resource and generate hydrogen in mass quantities and cheaper prices in order to compete with the traditional energy sources. There are three main methods that scientists are researching for inexpensive hydrogen generation. All three separate the hydrogen from a 'feedstock', such as fossil fuel or water - but by very different means.
Reformers - Fuel cells generally run on hydrogen, but any hydrogen-rich material can serve as a possible fuel source. This includes fossil fuels – methanol, ethanol, natural gas, petroleum distillates, liquid propane and gasified coal. The hydrogen is produced from these materials by a process known as reforming. This is extremely useful where stored hydrogen is not available but must be used for power, for example, on a fuel cell powered vehicle. One method is endothermic steam reforming. This type of reforming combines the fuels with steam by vaporizing them together at high temperatures. Hydrogen is then separated out using membranes. One drawback of steam reforming is that is an endothermic process – meaning energy is consumed. Another type of reformer is the partial oxidation (POX) reformer. CO2 is emitted in the reforming process, which makes it not emission-free, but the emissions of NOX, SOX, Particulates, and other smog producing agents are probably more distasteful than the CO2. And fuel cells cut them to zero.
Enzymes - Another method to generate hydrogen is with bacteria and algae. The cyanobacteria, an abundant single-celled organism, produces hydrogen through its normal metabolic function,. Cyanobacteria can grow in the air or water, and contain enzymes that absorb sunlight for energy and split the molecules of water, thus producing hydrogen. Since cyanobacteria take water and synthesize it to hydrogen, the waste emitted is more water, which becomes food for the next metabolism.
Solar- and Wind- powered generation - By harnessing the renewable energy of the sun and wind, researchers are able to generate hydrogen by using power from photovoltaics (PVs), solar cells, or wind turbines to electrolyze water into hydrogen and oxygen. In this manner, hydrogen becomes an energy carrier – able to transport the power from the generation site to another location for use in a fuel cell. This would be a truly zero-emissions way of producing hydrogen for a fuel cell.
Other Methods of Hydrogen Generation
Biomass Gasification and Pyrolysis
Biomass can be utilized to produce hydrogen. The biomass is first converted into a gas through high-temperature gasifying, which produces a vapor. The hydrogen rich vapor is condensed in pyrolysis oils and then can be steam reformed to generate hydrogen. This process has resulted in hydrogen yields of 12% - 17% hydrogen by weight of the dry biomass. The feedstock for this method can consist of wood chips, plant material, agricultural and municipal wastes, etc… When biological waste material is used as a feedstock, this method of hydrogen production becomes a completely renewable, sustainable method of hydrogen generation.
Fuel Cell Environmental Benefits
High Fuel Efficiencies
- By converting fuel directly into energy through an electrochemical reaction, fuel cells extract more power out of the same quantity of fuel when compared to traditional combustion. This direct process results in a reduced amount of fuel being consumed and greater efficiencies, 30% to 90%, depending on the fuel cell system and if the surplus heat is utilized. Combustion-based energy generation first converts the fuel into heat, limited by Carnot's Law of Thermodynamics, and then into mechanical energy, which provides motion or drives a turbine to produce energy. The additional steps involved in combustion generation allow energy to escape as heat, friction and conversion losses, resulting in lower overall efficiencies.
- When hydrogen is the fuel; water, heat and electricity are the by-products of the electrochemical reaction in a fuel cell generating electricity, instead of carbon dioxide, nitrogen oxides, sulfur oxides and particulate matter inherent to fossil fuel combustion.
When fossil fuels are reformed into hydrogen, emissions of carbon dioxide, nitrous oxides, sulfur oxides and other pollutants are a fraction of those produced through the combustion of the same amount of fuel.
- Fuel cells avoid the environmental damage associated with the extraction of fossil fuels from the Earth when the hydrogen is produced from renewable sources. If a hydrogen spill occurred, it would evaporate instantly, because hydrogen is lighter than air, leaving only water behind. This a dramatic departure from the legacy that oil drilling, transportation, refining and waste products have left on the Earth. How does a Fuel Cell work?
 Electrochemical fuel cells convert the chemical energy of fuels directly into electrical energy to provide a clean and highly efficient source of electrical energy, potentially to power electric vehicles. Although fuel cell research dates back at least 30 years, nearly all large automakers recently have begun projects to develop and evaluate fuel cell-powered vehicles. Their goals are reduced costs, minimal pollution and high efficiency. Like a battery, a fuel cell consists of two electrodes separated by an electrolyte made of a thin polymeric membrane. But unlike a battery, a fuel cell doesn't need recharging. It will continue to produce electricity as long as fuel flows through it.
In a fuel cell, hydrogen gas from the fuel reacts electrochemically at one electrode and converts into protons and electrons. The protons move through the electrolyte to the other electrode, where they combine with oxygen from the air and with the electrons to form water, which is expelled from the cell as vapor. The involvement of hydrogen and oxygen in the two reactions - one releasing electrons and the other consuming them - yields electrical energy that is tapped across the electrodes for power, for example, to drive a motor.
Highly efficient fuel cells based on polymer electrolyte catalysts, known as proton-exchange membrane fuel cells, were developed by General Electric for the Gemini space program, but required large amounts of a costly platinum catalyst. The heart of the PEM fuel cell is a polymer membrane that has thin films of catalyst bonded on both its major surfaces, providing effective catalytic sites for the electrode processes.
Highly efficient fuel cells based on polymer electrolyte catalysts, known as proton-exchange membrane fuel cells, were developed by General Electric for the Gemini space program, but required large amounts of a costly platinum catalyst. The heart of the PEM fuel cell is a polymer membrane that has thin films of catalyst bonded on both its major surfaces, providing effective catalytic sites for the electrode processes.
courtesy of Ecosoul
Electrochemical fuel cells convert the chemical energy of fuels directly into electrical energy to provide a clean and highly efficient source of electrical energy, potentially to power electric vehicles. Although fuel cell research dates back at least 30 years, nearly all large automakers recently have begun projects to develop and evaluate fuel cell-powered vehicles. Their goals are reduced costs, minimal pollution and high efficiency. Like a battery, a fuel cell consists of two electrodes separated by an electrolyte made of a thin polymeric membrane. But unlike a battery, a fuel cell doesn't need recharging. It will continue to produce electricity as long as fuel flows through it.
In a fuel cell, hydrogen gas from the fuel reacts electrochemically at one electrode and converts into protons and electrons. The protons move through the electrolyte to the other electrode, where they combine with oxygen from the air and with the electrons to form water, which is expelled from the cell as vapor. The involvement of hydrogen and oxygen in the two reactions - one releasing electrons and the other consuming them - yields electrical energy that is tapped across the electrodes for power, for example, to drive a motor.
Highly efficient fuel cells based on polymer electrolyte catalysts, known as proton-exchange membrane fuel cells, were developed by General Electric for the Gemini space program, but required large amounts of a costly platinum catalyst. The heart of the PEM fuel cell is a polymer membrane that has thin films of catalyst bonded on both its major surfaces, providing effective catalytic sites for the electrode processes.
Highly efficient fuel cells based on polymer electrolyte catalysts, known as proton-exchange membrane fuel cells, were developed by General Electric for the Gemini space program, but required large amounts of a costly platinum catalyst. The heart of the PEM fuel cell is a polymer membrane that has thin films of catalyst bonded on both its major surfaces, providing effective catalytic sites for the electrode processes.
courtesy of Ecosoul
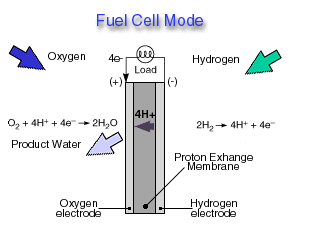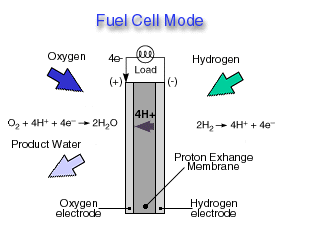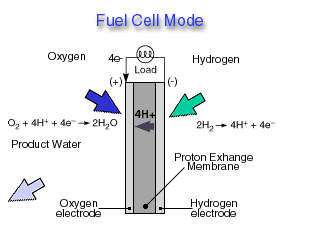 Through a single electrochemical process, a fuel cell produces electricity, water, and heat using fuel and oxygen in the air . Water is the only emission when hydrogen is the fuel. As hydrogen flows into the fuel cell on the anode side (see Fuel Cell Mode figure), a platinum catalyst facilitates the separation of the hydrogen gas into electrons and protons (hydrogen ions) in a proton exchange membrane or PEM fuel cell.
The hydrogen ions pass through the membrane (the center part of a PEM fuel cell) and, again with the help of a platinum catalyst, combine with oxygen and electrons on the cathode side producing water. The electrons, which cannot pass through the membrane, flow from the anode to the cathode through an external circuit containing an electric load which consumes the power generated by the cell. The overall electrochemical process of a fuel cell is called "reverse hydrolysis," or the opposite of hydrolyzing water to form hydrogen and oxygen.
Through a single electrochemical process, a fuel cell produces electricity, water, and heat using fuel and oxygen in the air . Water is the only emission when hydrogen is the fuel. As hydrogen flows into the fuel cell on the anode side (see Fuel Cell Mode figure), a platinum catalyst facilitates the separation of the hydrogen gas into electrons and protons (hydrogen ions) in a proton exchange membrane or PEM fuel cell.
The hydrogen ions pass through the membrane (the center part of a PEM fuel cell) and, again with the help of a platinum catalyst, combine with oxygen and electrons on the cathode side producing water. The electrons, which cannot pass through the membrane, flow from the anode to the cathode through an external circuit containing an electric load which consumes the power generated by the cell. The overall electrochemical process of a fuel cell is called "reverse hydrolysis," or the opposite of hydrolyzing water to form hydrogen and oxygen.
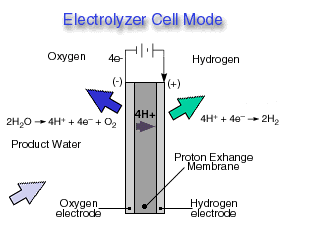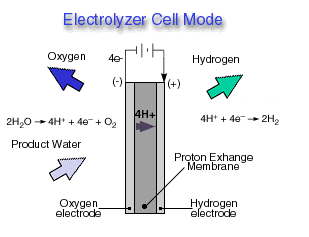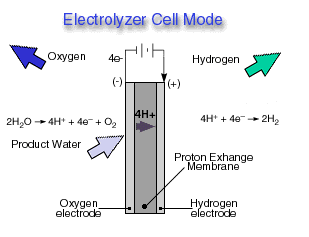 A reversible fuel cell can accomplish "hydrolysis" through the supply of electricity to the cell and a supply of water to the cathode (see Electrolyzer Cell Mode ). Only certain fuel cell types are reversible, that is, can also accomplish the electrochemistry associated with both the production of electricity from fuel and oxidant and the production of fuel and oxidant from water when supplied with electricity.
The Reversible fuel cell concept is one that incorporates a reversible fuel cell that can accomplish both hydrolysis and reverse hydrolysis in the same cell. This allows one to consider the completely renewable production of electricity by using a renewable energy supply (e.g., solar, wind) to produce hydrogen and oxygen from water which can subsequently be used to produce electricity through the same fuel cell from the fuel and oxidant produced previously.
All text and diagrams courtesy of Ecosoul.
A reversible fuel cell can accomplish "hydrolysis" through the supply of electricity to the cell and a supply of water to the cathode (see Electrolyzer Cell Mode ). Only certain fuel cell types are reversible, that is, can also accomplish the electrochemistry associated with both the production of electricity from fuel and oxidant and the production of fuel and oxidant from water when supplied with electricity.
The Reversible fuel cell concept is one that incorporates a reversible fuel cell that can accomplish both hydrolysis and reverse hydrolysis in the same cell. This allows one to consider the completely renewable production of electricity by using a renewable energy supply (e.g., solar, wind) to produce hydrogen and oxygen from water which can subsequently be used to produce electricity through the same fuel cell from the fuel and oxidant produced previously.
All text and diagrams courtesy of Ecosoul.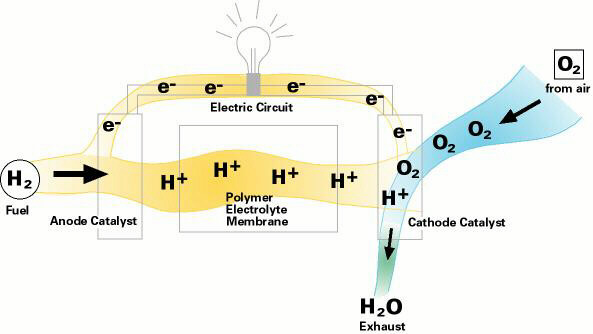
Fuel Flexibility
- Fuel cells are capable of operating on hydrogen, or hydrogen reformed from any of the common fossil fuels available today.
- The amount of power a fuel cell can generate within a given volume is usually given in kWh/liter. These numbers continue to rise as manufacturers continue research and development on their respective products.
- Fuel cells operate at 80o C to over 1,000o C, depending on the type of fuel cell. These numbers might seem high, but the temperature inside your vehicle's internal combustion engine can reach over 2,300o C.
- Fuel cells, with their inherently quiet operation, zero to minimal emissions and reduced permitting requirements, can be located in a variety of areas, both residential and commercial, inside and outside.
- When the waste heat from the fuel cell's electrochemical reaction is captured, it can be utilized for water, space heating and cooling. With cogeneration capabilities, the efficiencies achieved by a fuel cell system approach 90%.
- To receive additional energy from a fuel cell, more fuel is introduced into the system. Fuel cell load response is analogous to depressing the gas pedal in your vehicle, more fuel more power.
A residential fuel cell system allows people to become independent of the brown outs, power failures and voltage irregularities that are commonplace when connected to the utility grid. Any one of these common power disruptions can damage sensitive computer systems, electronic equipment and the quality of life people desire to have.
Reliable energy in areas that are subjected to weather related power outages.
Fuel Cells vs. Traditional Batteries
 Fuel cells offer a reduction in weight and come in a compact package for the same amount of available energy when compared to batteries.
Fuel cells offer a reduction in weight and come in a compact package for the same amount of available energy when compared to batteries. To increase the power in a fuel cell, more fuel is introduced into the system. To increase the power of a battery, more batteries have to be added increasing the cost, weight and complexity of the system.
A fuel cell never "runs down", it continues to produce electricity as long as fuel is present. When a battery "runs down" it has to undergo a lengthy, inconvenient recharge time to replace the spent electricity. Depending on where the electricity originates, pollution, costs and efficiency problems are transferred from the batteries location to the central generating plant.
Phosphoric Acid. This type of fuel cell is commercially available today. More than 200 fuel cell systems have been installed all over the world - in hospitals, nursing homes, hotels, office buildings, schools, utility power plants, an airport terminal, even a municipal waste dump. Phosphoric acid fuel cells generate electricity at more than 40% efficiency -- and nearly 85% of steam this fuel cell produces is used for cogeneration -- this compares to about 35% for the utility power grid in the United States. Operating temperatures are in the range of 400 degrees F.
 Proton Exchange Membrane. These cells operate at relatively low temperatures (about 200 degrees F), have high power density, can vary their output quickly to meet shifts in power demand, and are suited for applications, -- such as in automobiles -- where quick startup is required. According to the U.S. Department of Energy, "they are the primary candidates for light-duty vehicles, for buildings, and potentially for much smaller applications such as replacements for rechargeable batteries." The proton exchange membrane is a thin plastic sheet that allows hydrogen ions to pass through it. The membrane is coated on both sides with highly dispersed metal alloy particles (mostly platinum) that are active catalysts. Hydrogen is fed to the anode side of the fuel cell where the catalyst encourages the hydrogen atoms to release electrons and become hydrogen ions (protons). The electrons travel in the form of an electric current that can be utilized before it returns to the cathode side of the fuel cell where oxygen has been fed. At the same time, the protons diffuse through the membrane to the cathode, where the hydrogen atom is recombined and reacted with oxygen to produce water, thus completing the overall process.
Proton Exchange Membrane. These cells operate at relatively low temperatures (about 200 degrees F), have high power density, can vary their output quickly to meet shifts in power demand, and are suited for applications, -- such as in automobiles -- where quick startup is required. According to the U.S. Department of Energy, "they are the primary candidates for light-duty vehicles, for buildings, and potentially for much smaller applications such as replacements for rechargeable batteries." The proton exchange membrane is a thin plastic sheet that allows hydrogen ions to pass through it. The membrane is coated on both sides with highly dispersed metal alloy particles (mostly platinum) that are active catalysts. Hydrogen is fed to the anode side of the fuel cell where the catalyst encourages the hydrogen atoms to release electrons and become hydrogen ions (protons). The electrons travel in the form of an electric current that can be utilized before it returns to the cathode side of the fuel cell where oxygen has been fed. At the same time, the protons diffuse through the membrane to the cathode, where the hydrogen atom is recombined and reacted with oxygen to produce water, thus completing the overall process.Alkaline. Long used by NASA on space missions, these cells can achieve power generating efficiencies of up to 70 percent. They use alkaline potassium hydroxide as the electrolyte. Until recently they were too costly for commercial applications, but several companies are examining ways to reduce costs and improve operating flexibility.
Direct Methanol Fuel Cells. These cells are similar to the PEM cells in that they both use a polymer membrane as the electrolyte. However, in the DMFC, the anode catalyst itself draws the hydrogen from the liquid methanol, eliminating the need for a fuel reformer. Efficiencies of about 40% are expected with this type of fuel cell, which would typically operate at a temperature between 120-190 degrees F. Higher efficiencies are achieved at higher temperatures.
Regenerative Fuel Cells. Still a very young member of the fuel cell family, regenerative fuel cells would be attractive as a closed-loop form of power generation. Water is separated into hydrogen and oxygen by a solar-powered electrolyser. The hydrogen and oxygen are fed into the fuel cell which generates electricity, heat and water. The water is then recirculated back to the solar-powered electrolyser and the process begins again. These types of fuel cells are currently being researched by NASA and others worldwide.
No comments:
Post a Comment